NEEDLETECH
Needletech, hypodermic needles (Medical Device CE0476 of our production)
Size:
30G – 13mm
31G – 13mm
31G – 6mm
31G – 4mm
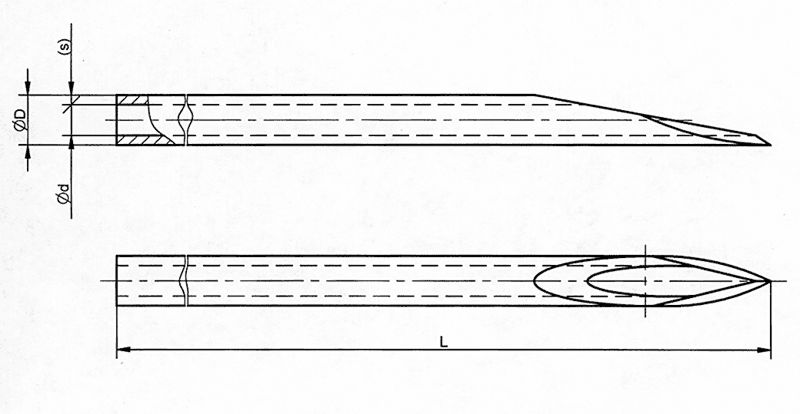
All the needles feature an acute angle needle tip in order to minimize the resistance to penetration into the skin and are polished using ultrasonic method.
This ensures a smooth needle's suface, free from any imperfections.
Manufactured using premium-quality stainless steel strip with the latest Korean technology.
Needle hubs are color coded to comply with ISO guidelines and this makes an easy identification of the relevant needle size.

 Main Field of Coverage:
Main Field of Coverage:
Mesotherapy
Botulinum Toxin
Sclerotherapy
Diabetes
(Size 31G da 6mm is also indicated for insulin pens).

GMDN:
An extremely fine, sharply-pointed metal tube
that is used in conjuction with syringes for puncture
into certain sites on the patient’s body, e.g., the eyeball,
for the introduction/extraction of very small quantities of
a medium, e.g., a body fluid, gas, or a medication.
Also known as a microlance, it will tipically have a standard
Luer-lock connector at the proximal end.
This is a single-use device.
93/42/EEC DIRECTIVE - Annex II, without point 4, transposed in Italy by Dlgs. 46 of 1997/02/24.
CE Classification: Class IIa
Technical Datas
Description: Hypodermic needle for the administration of
Injections and blood and body fluids to patient’s consumption.
Destination of use in accordance with D.lgs. 46/97: Professional
Presence of Substances/Tissues: NO
Presence of Drugs: NO
The main materials which constitute the primary packaging of
this MD, don’t require special conditions of disposal: NO
Sterile: YES
List of Sterilization Methods
Sterilization Method: ETHILENE OXIDE
Maximum period of use (months): 24
Sterilization Method validated in accordance to harmonized standards: NO
Single-use: YES
Latex – during manufactuing process the product came into contact with
Latex molecules: NO
Latex – both the product and its packaging are free of latex: YES
Materials which constitute the MD at direct contact with the Patient
Material: STAINLESS STEEL
Special condition of disposal: YES
List of Packaging’s materials:
PAPER/PLASTIC LAMINATE COMBINATION
Download Conformity CE
|
|











