NEEDLEFLEX
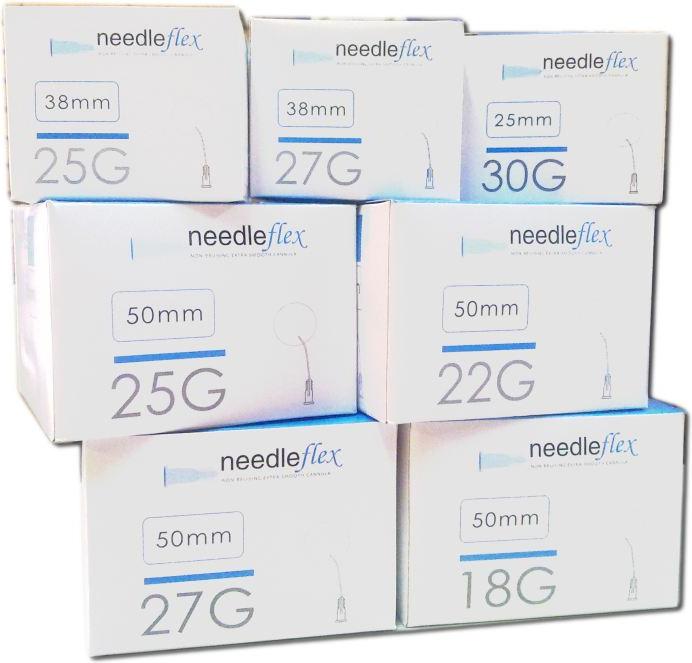 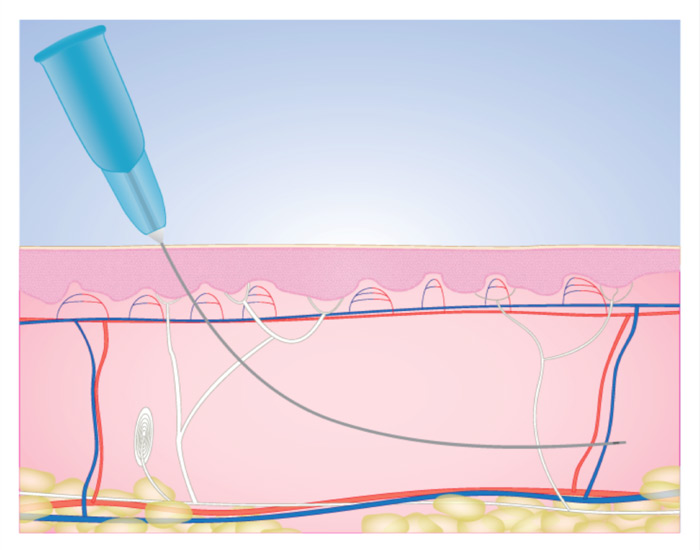 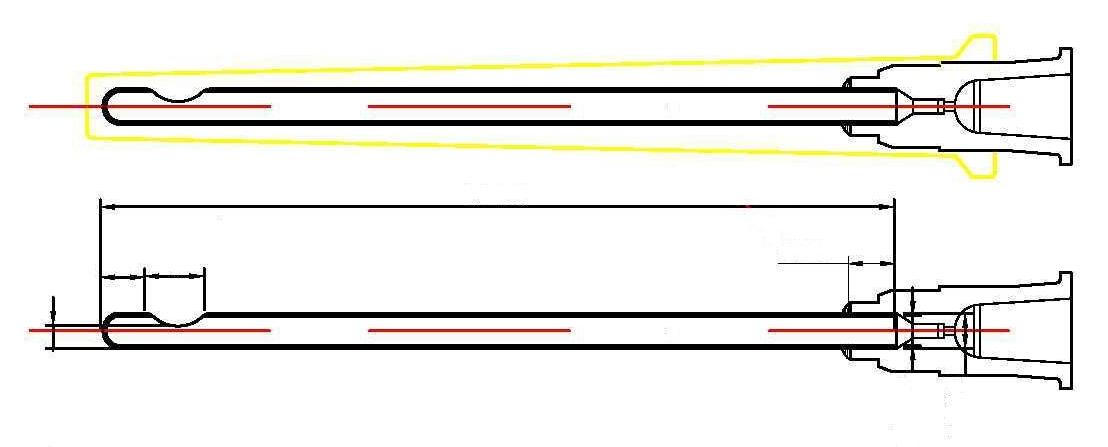
Needleflex, hypodermic cannula needles bluunt tip and side hole [Medical Device CE0476]
Size:
30G - 25mm (+ 26Gintroducer needles)
27G - 38mm (+ 23G introducer needles)
27G - 50mm (+ 23G introducer needles)
25G - 38mm (+ 23G introducer needles)
25G - 50mm (+ 23G introducer needles)
22G - 100mm (+ 18G introducer needles)
22G - 50mm (+ 18G introducer needles)
18G - 100mm (+ 18G introducer needles)
18G - 50mm (+ 16G introducer needles)
GMDN:
An extremely fine, sharply-pointed metal tube
that is used in conjuction with syringes for puncture
into certain sites on the patient’s body, e.g., the eyeball,
for the introduction/extraction of very small quantities of
a medium, e.g., a body fluid, gas, or a medication.
Also known as a microlance, it will tipically have a standard
Luer-lock connector at the proximal end.
This is a single-use device.
93/42/EEC DIRECTIVE - Annex II, without point 4, transposed in Italy by Dlgs. 46 of 1997/02/24.
CE Classification: Class IIa
Technical Datas
Description: Hypodermic needle for the administration of
Injections and blood and body fluids to patient’s consumption.
Destination of use in accordance with D.lgs. 46/97: Professional
Presence of Substances/Tissues: NO
Presence of Drugs: NO
The main materials which constitute the primary packaging of
this MD, don’t require special conditions of disposal: NO
Sterile: YES
List of Sterilization Methods
Sterilization Method: ETHILENE OXIDE
Maximum period of use (months): 24
Sterilization Method validated in accordance to harmonized standards: NO
Single-use: YES
Latex – during manufactuing process the product came into contact with
Latex molecules: NO
Latex – both the product and its packaging are free of latex: YES
Materials which constitute the MD at direct contact with the Patient
Material: STAINLESS STEEL
Special condition of disposal: YES
List of Packaging’s materials:
PAPER/PLASTIC LAMINATE COMBINATION
Download Conformity CE
|
|











